Research Park at Florida Atlantic University® Based FloSpine Receives FDA Clearance for PANAMA™ Medical Device

MEDIA CONTACT: Andrew Duffell
561-416-6092 Ext. 1402, aduffell@Research-Park.org
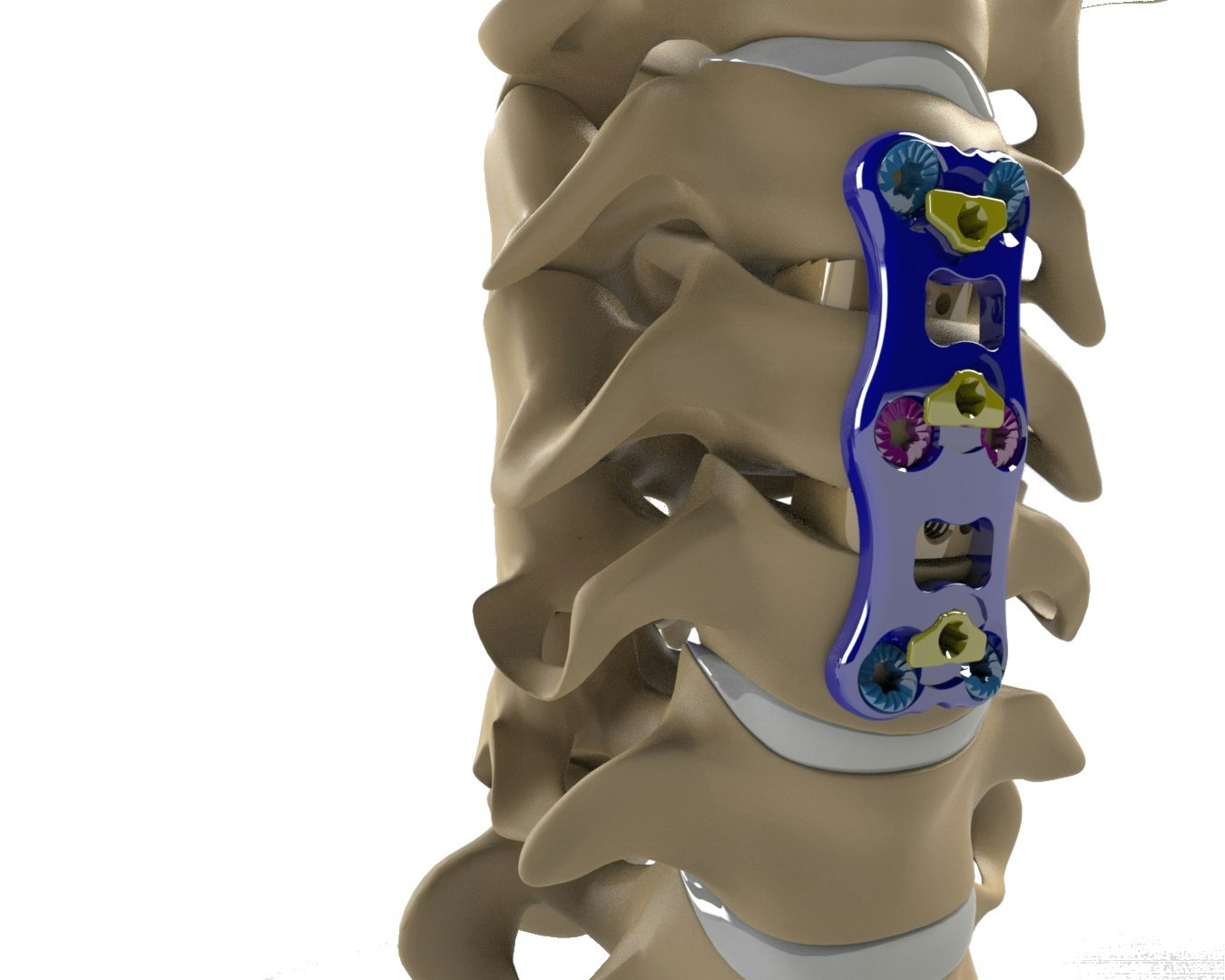
BOCA RATON, Fla. (May 20, 2021) – Together with FloSpine, LLC, the Research Park at Florida Atlantic University® is pleased to announce that FloSpine has achieved 510(k) clearance for its PANAMA™ Anterior Cervical Plate System from the Food and Drug Administration (FDA).
The PANAMA™ Anterior Cervical Plate is used as supplemental fixation to support the spine after a cervical discectomy and fusion has been performed. The system is designed with a unique screw locking mechanism that prevents screw backout after being implanted in a patient.
FloSpine is a spinal implant company that researches and develops innovative and cost-effective medical devices for spinal implants and instruments. The company's goal is to apply new technology and design concepts to solve medical problems of the human spinal column. The company provides surgeons with products resulting in enhanced surgical outcomes, shorter hospital stays, and quicker recovery times, thereby reducing the procedural and rehabilitative costs to the patient and insurer.
Participating in the Research Park at FAU’s Global Ventures second-stage entrepreneurial support initiative has enabled the company to access highly qualified and motivated graduate students from FAU’s College of Engineering and Computer Science who have become full-time employees, access to specialized equipment at Florida Atlantic University and connections to its research faculty. Global Ventures has nominated FloSpine to the Florida Companies to Watch award, hosted by GrowFL.
Dr. John Afshar, MD, neurosurgical spine surgeon at Palm Beach Neuroscience Institute, West Palm Beach, FL commented “The PANAMA Cervical Plate system is unique with its double locking mechanism and extreme bone screw angulation. This gives a surgeon the confidence that the cervical plate is secure and will improve patient outcomes”.
“I was very gratified to see many of our recommendations and ideas that were used in the refinement of the system and we look forward to using the product to improve patient care”, said Dr. John Robinson, Jr., MD, spine surgeon at Palm Beach Neuroscience Institute. Both Dr. Robinson and Dr. Afshar are two of the area’s highly experienced, board certified neurosurgeons specializing in treating multiple disorders of the spine.
The PANAMA Anterior Cervical Plate Systems adds to the existing portfolio of 3 products designed for complex spine disorders, including scoliosis and degenerative disc disease. The previously cleared products are the Canaveral Pedicle Screw System and Largo Interbody Spacer System.

-END-
About FloSpine, LLC
FloSpine, LLC is a privately held US medical device company offering products for addressing complex deformity spine problems, minimally invasive spine surgery and thoracolumbar degenerative conditions. For more information, please visit FloSpine at http://www.flospine.com. Statements made in this press release that look forward in time or that express beliefs, expectations or hopes regarding future occurrences or anticipated outcomes are forward-looking statements. A number of risks and uncertainties such as risks associated with product development and commercialization efforts, expected timing or results of any clinical trials, ultimate clinical outcome and perceived or actual advantages of the Company’s products, market and physician acceptance of the products, intellectual property protection, and competitive offerings could cause actual events to adversely differ from the expectations indicated in these forward-looking statements.
About the Research Park at Florida Atlantic University®
The Research Park at Florida Atlantic University® is home to technology companies and research-based organizations working to support the research and development activities of Florida Atlantic University and to foster economic development and broaden the economic base of Broward and Palm Beach counties. The Research Park at FAU hosts Global Ventures, an international soft-landing center for second-stage technology companies and FAU Tech Runway, a South Florida public-private partnership that serves as a hub to accelerate technology development and incubate startup companies. The Research Park at FAU is a 70-acre destination for R&D companies to thrive, established in 1985, it is widely regarded as South Florida’s laboratory for new entrepreneurial ideas and technologies. The Research Park at FAU is governed by the Florida Atlantic Research and Development Authority, an independent special district created by Palm Beach and Broward counties in partnership with Florida Atlantic University, organized under Chapter 159, Part V, Florida statues.
